Abstract
Introduction: Conflicting results from two recently reported randomized studies comparing double vs single autotransplantation (ASCT) for newly diagnosed multiple myeloma (MM) patients (pts) [(Cavo M et al, Blood 2017;130(1); Stadtmauer EA et al, Blood 2016;128(22)] are likely to reflect differences in the design of the trials. To address this controversial issue, we performed a long-term follow-up analysis of pt-level data from three phase 3 trials of bortezomib-thalidomide-dexamethasone (VTD) (Cavo M et al, Lancet 376; 2075-85, 2010; Rosinol L et al, Blood 120; 1589-96, 2012) or bortezomib-doxorubicin-dexamethasone (PAD) (Sonneveld P et al, J Clin Oncol 30; 2946-55, 2012) as induction therapy before ASCT, followed by post-ASCT bortezomib-based consolidation and/or maintenance treatment. According to study design, patients were assigned to receive either a single or double ASCT (ASCT-1 or ASCT-2), thus allowing a comparison between these treatments.
Methods: The intent-to-treat population included 909 pts who were randomized to either VTD or PAD arms of the studies and for whom ASCT-1 (n=501) or ASCT-2 (n=408) were planned at study entry. Median age was 58 yrs in both groups; the rate of ISS stage III was 20% and 17%, respectively, while 18% and 23% of pts in ASCT-1 and ASCT-2 groups were positive for t(4;14) and/or del(17p) (cut-off levels ≥10% and ≥20%, respectively) by FISH analysis.
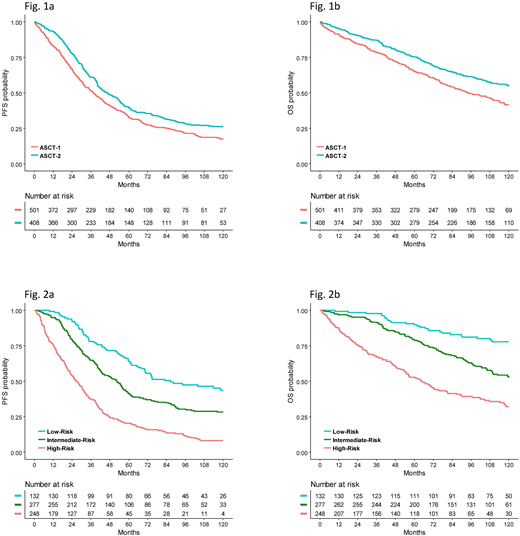
Results: With a median follow up of 117 mos (IQR 91-126), assignment to ASCT-2 resulted in superior PFS (median: 47 vs 38 mos; HR 0.76, 95%CI=0.65-0.89, p=0.0008) and OS (estimated 10-yr probability: 58% vs 47%; HR 0.69, CI 0.56-0.84, p=0.0002) in comparison with ASCT-1 (Figure 1). PFS benefit with ASCT-2 was retained across prespecified subgroups, including pts with both standard-risk (median: 53 vs 43 mos; HR 0.74, CI 0.61-0.91, p=0.005) and high-risk cytogenetics (cyto) (median: 36 vs 20 mos; HR 0.67, CI 0.46-0.97, p=0.032). The 10-yr OS rates were 72% with ASCT-2 vs 60% with ASCT-1 (HR 0.68, CI 0.52-0.88, p=0.004) for pts with standard-risk and 51% vs 34% (HR 0.54, CI 0.36-0.83, p=0.004) for those with high-risk cyto. In a multivariate Cox regression analysis, independent predictors for prolonged PFS included ASCT-2 (HR 0.81, CI 0.66-0.99, p=0.048), platelet (PLT) count >150.000/mmc (HR 0.74, CI 0.54-0.99, p=0.049), ISS stage I+II (HR 0.62, CI 0.48-0.80, p<0.001), absence of t(4;14) and/or del(17p) (HR 0.57, CI 0.45-0.73, p<0.001), and complete response (CR) recorded at any time throughout treatment (best CR) (HR 0.53, CI 0.43-0.64, p<0.001). These variables were also significantly related to longer OS (ASCT-2: HR 0.75, CI 0.57-0.98, p=0.036; PLTs: HR 0.55, CI 0.38-0.78, p=0.001; ISS stage: HR 0.66, CI 0.48-0.91, p=0.010; cyto: HR 0.55, CI 0.41-0.73, p<0.001; best CR: HR 0.55, CI 0.43-0.72, p<0.001).
HR estimates of the leading, not including therapy, predictors of outcomes (ISS stage II+III, high-risk cyto and failure to achieve best CR) were used to build a score index that stratified patients into 3 subgroups at low-risk (20%, none of the 3 adverse variables), intermediate-risk (42%, 1 adverse variable) and high-risk (38%, 2 or 3 adverse variables). Median PFS for these subgroups was 87, 53 and 27 mos (p<0.001), while the corresponding 10-yr OS rates were 78%, 53% and 32% (p<0.001) (Figure 2). There was a trend to improved PFS, but not OS, with ASCT-2 vs ASCT-1 in the low-risk subgroup (53% vs 28% at 10 yrs; HR 0.66, CI 0.66-0.41, p=0.093). Conversely, in the high-risk subgroup assignment to ASCT-2 significantly prolonged both PFS (median: 32 vs 20 mos, HR 0.71, CI 0.54-0.93, p=0.012) and OS (43% vs 20% at 10 yrs; HR 0.58, CI 0.42-0.80; p=0.001) in comparison with ASCT-1. Notably, the greatest benefit from ASCT-2 was observed in the ultra high-risk subset of pts with 3 adverse variables who enjoyed a two-fold increased PFS (median: 35 vs 14 mos; HR 0.45, CI 0.21-0.79; p=0.008) and 56% reduction in the risk of death (26% vs 6% estimated 10-yr OS probability; HR 0.44, CI 0.21-0.90; p=0.025) compared with ASCT-1.
Conclusions: Results of this pooled analysis of phase 3 studies incorporating bortezomib-based triplets into ASCT confirmed the superiority of ASCT-2 over ASCT-1 in terms of extended PFS and OS. The subgroup of pts at high-risk mostly benefited from ASCT-2, in particular those who had advanced ISS stage, adverse cyto and failed to achieve CR.
Cavo:GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Goldschmidt:Celgene: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Research Funding; Adaptive Biotechnology: Consultancy; Sanofi: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Chugai: Honoraria, Research Funding; Mundipharma: Research Funding; Novartis: Honoraria, Research Funding; ArtTempi: Honoraria; Janssen: Consultancy, Honoraria, Research Funding. Rosinol:Janssen, Celgene, Amgen, Takeda: Honoraria. Zweegman:Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene Corp.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Salwender:Novartis: Honoraria, Other: travel suppport, Research Funding; Amgen: Honoraria, Other: travel suppport, Research Funding; Takeda: Honoraria; Bristol-Myers Squibb: Honoraria, Other: travel suppport, Research Funding; Janssen: Honoraria, Other: travel support, Research Funding; Celgene: Honoraria, Other: travel suppport, Research Funding. Lahuerta:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Petrucci:Bristol-Myers Squibb: Honoraria, Other: Advisory Board; Takeda: Honoraria, Other: Advisory Board; Amgen: Honoraria, Other: Advisory Board; Celgene: Honoraria, Other: Advisory Board; Janssen-Cilag: Honoraria, Other: Advisory Board. Oriol:Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Weisel:Amgen, Celgene, Janssen, and Sanofi: Research Funding; Amgen, BMS, Celgene, Janssen, and Takeda: Honoraria; Amgen, BMS, Celgene, Janssen, Juno, Sanofi, and Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees. Rios:Amgen, Celgene, Janssen, and Takeda: Consultancy. Patriarca:Celgene: Other: Advisory Role; Travel, accommodations, expenses; Medac: Other: Travel, accommodations, expenses; Jazz: Other: Travel, accommodations, expenses; Janssen: Other: Advisory role; MSD Italy: Other: Advisory Role. Mateos:GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Galli:Sigma-Tau: Honoraria; Celgene: Honoraria; Janssen: Honoraria; Bristol-Myers Squibb: Honoraria. San-Miguel:Novartis: Honoraria; Sanofi: Honoraria; Roche: Honoraria; BMS: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Celgene: Honoraria. Boccadoro:Novartis: Honoraria, Research Funding; AbbVie: Honoraria; Janssen: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Mundipharma: Research Funding; Sanofi: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding. Blade:Janssen: Honoraria; Celgene: Honoraria; Amgen: Honoraria. Sonneveld:Amgen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Karyopharm: Honoraria, Research Funding; BMS: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal